Localized,nonlinear,waves,in,a,myelinated,nerve,fiber,with,self-excitable,membrane
Nkeh Oma Nfor Patrick Guemkam Ghomsi and Francois Marie Moukam Kakmeni
1Department of Physics,HTTC Bambili,University of Bamenda,P.O.Box 39 Bambili,Cameroon
2Complex Systems and Theoretical Biology Group,Laboratory of Research on Advanced Materials and Nonlinear Science(LaRAMaNS),Department of Physics,Faculty of Science,University of Buea,P.O.Box 63 Buea,Cameroon
Keywords: myelinated nerve, Fitzhugh-Nagumo, capacitive feedback parameter, Ginzburg-Landau, collision,annihilation
There is a growing interest in recent years to investigate the nonlinear processing of information in reaction-diffusion systems, with sharp focus on neural networks.[1-6]From a biophysical perspective,the knowledge of neural information propagation mechanism constitutes one of the most interesting scientific challenges in excitable media.Generally speaking,an excitable system is one in which an initial stimulus of sufficient amplitude initiates a traveling wave propagating through the medium.[7,8]The mechanism of nerve impulse propagation was first investigated by Hodgkin-Huxley in 1952,[9,10]based on data from the squid giant axon for the space clamp membrane.This ionic model is highly physiological and governed by four nonlinear differential equations.Due to some computational difficulties encountered,Richard FitzHugh and Jin-ichi Nagumo independently proposed a simplified neuronal model of Hodgkin and Huxley known as the FitzHugh-Nagumo(FHN)model.[11,12]The FHN model is based on the Van Der Pol equations and mainly captures and crystallizes all the features of a dynamical system.
Concretely in the 1940s, Hodgkin, Huxley, and Katz mathematically and experimentally investigated the nature of nerve impulses.Their research revealed that the electrical pulses across the membrane arise from the uneven distribution between the intracellular fluid and the extracellular fluid of potassium(K+),sodium(Na+),and protein anions.[9,10,13]When a neuron is not emitting a signal,it is said to be at rest of roughly-70 mV and the interior part of the neuron is more negative with respect to the exterior.The variation in the Na+and K+permeability allows for the movement of ions in and out of the cell by means of opening and closing of various ion channels.The influx of Na+and efflux of K+results in electrical potential difference.Triggered by a stimulus the Na+channels open,the influx of Na+ions increases,the membrane depolarizes,and the potential voltage reaches a threshold level typically between-50 mV and-55 mV.At this moment an abrupt depolarization takes place,which rapidly moves the potential to a maximum of +30 mV.[9,10,13]At a similar rate,the Na+and K+channels close and open,respectively,initiating membrane repolarization caused by an efflux of K+ions,thereby causing the potential to drop back to resting state.During the repolarization process, the membrane goes through a transient hyperpolarization of-10 mV below resting potential.In a nutshell,all these changes in potential are physically depicted as an action potential,impulse or spike observed during the transmission of nerve impulses.
The above description of the action potential based on the Hodgkin-Huxley(HH)model is limited because it mainly assumes that the membrane capacitance is a constant.[9,10]However, experimental results suggest that the changes in membrane capacitance are of an order of magnitude sufficient to account for the observed voltage changes during the action potential.[14,15]This variation in membrane capacitance may emanate from changes in thickness and area (among other physiological factors) of the membrane during the action potential.Recent studies have been carried out to model the membrane capacitance as a nonlinear function of the transmembrane voltage (see, for example, Ref.[6] and references therein).Dikand´eet al.[6]considered two types of capacitance-voltage (C-V) characteristics, which are polynomial functions of the transmembrane voltage, and showed that the cytoplasm behaves like a self generative excitable medium during the transmission of nerve impulses.They further demonstrated that for both myelinated and unmyelinated nerve fibers, the nerve impulses are well-localized in spacetime coordinates with their shapes and stability determined by theC-Vcharacteristics.However, the present study clearly differs from the one carried out by Dikand´eet al.in the following three ways: (i)We focus mainly on a myelinated axon with a recovery mechanism.(ii) The ionic current is considered to be a nonlinear function of the transmembrane voltage.(iii) The issue of dissipation in the nerve fiber is addressed,which enables us to monitor the evolution of nerve impulse without significant distortion.Here the effects of dissipation clearly dominates that of linear dispersion, leading to a nonlinear diffusive process because of the interplay between nonlinearity and dissipation.Traveling pulses in dissipative systems is some times termed dissipative solitons, which were first predicted to exist in reaction-diffusion systems.[16]Dissipative and nonlinear effects are also crucial factors in reactiondiffusion systems,with the dissipative effect spreading though out a neural network because of the spatially localized connectivity between adjacent group of neurons.However, the dynamic balance between these two components usually generates diffusive solitons observed in most excitable media.
The conduction of action potential is physically manifested as the propagation of nerve impulse that maintains its amplitude, density, and velocity during the transmission of neuronal information along a conservative axon.The phenomenon of modulational instability, which is triggered by competitive effects of nonlinearity and dispersion in nonlinear systems,[17-20]is inextricably linked to the observation of soliton-like modes in neural networks.[1,4,5]Ever since the discovery of solitons by Fermi,Pasta,and Ulam,[17]enormous efforts have been made to elucidate on many complex phenomena in a plethora of nonlinear physical systems.This ranges from the propagation of femtosecond pulses in erbium doped fibers,[21]observation of first-order bright solitary waves in non-autonomous generalized AB system,[22,23]classification of solutions to the defocusing complex modified Korteweg-de Vries equation with the step-like initial condition,[24]among many others.
This study mainly presents an electrical diffusive lattice in the form of a transmission line that models the modified discrete FHN equations, which physically depicts a myelinated axon with nonlinear membrane capacitance.The mathematical study of spatially discrete models is challenging because of special and poorly understood phenomena occurring in them like propagation failure.In fact, this work is an extension of the one carried out in Ref.[4], with the exception that the membrane capacitance of the myelinated axon is now a polynomial function of transmembrane voltage.Hence this membrane is considered as a self-excitable organ with capacitive feedback parameter,α, used to modify the dynamics of the propagating modulated nerve impulses.We will thus derive the complex Ginzburg-Landau (CGL) amplitude equation from the modified discrete FHN model with the system of equation reduced to the dissipative nonlinear Schr¨odinger(DNLS)equation whenα=αcr(whereαcris the critical value of the capacitive feedback parameter).Bright solitary wave solutions of the DNLS equation are obtained,which enable us to observe the propagation of modulated nerve impulses in the myelinated axon at this critical value.
In Section 2, we describe an electrical circuit that represents a discrete FHN model with nonlinear membrane capacitance.By using Kirchhoff’s current and voltage laws,we derive the modified discrete FHN equation which is transformed to its Li´enard form.The model CGL amplitude equation is eventually obtained from the Li´enard equation using the multiple scale expansion in the semi-discrete approximation perturbation method.Modulational instability analysis is carried in Section 3, with results that fulfill the Benjamin-Feir criteria.This clearly shows that the modulated nerve impulses emanate from unstable plane wave solutions,with variation inαgenerally influencing the stability of plane waves.Analytic solutions of the derived amplitude equations lead to the observation of modulated nerve impulses.A numerical simulation scheme of the Li´enard form of the modified discrete FHN equation is carried out in Section 4,with initial condition being the solutions of the derived amplitude equations.Results of the numerical simulations mainly confirm the propagation,collision,and annihilation of nerve impulses in the myelinated axon,as the various relevant parameters are varied.Finally the main results are summarized in Section 5 with discussion and conclusion.
We consider a nonlinear electrical FHN lattice realized withNelementary cells,resistively coupled by linear resistorsRc, as represented in Fig.1.Each cell contains a nonlinear capacitor of capacitanceC(Un),a linear self-inductanceL,linear resistorR, a cellε, and a nonlinear resistor of resistanceRNL.The current-voltage characteristic of the nonlinear resistor obeys the following cubic law:[11,12]
whereR0andU0are normalization parameters withR0being a weighting resistor.This nonlinear resistorRNLis mostly constructed using analogue multipliers.

Fig.1.Schematic representation of the electrical lattice under consideration.The nonlinear resistance RNL and nonlinear capacitance C(Un)are functions of the transmembrane voltage.
Using Kirchhoff’s laws leads to the set of differential equations describing nonlinear propagation in the lattice,
whereUnandiL,nare the voltage and the resulting current,respectively, through the linear inductorLat thenth cell.By using the normalized variables:un=Un/U0,K=R0/Rc,vn=R0iL,n/U0,a=R20ε/LU20,b=R20/LU0,c=RR0/LU0andτ=U0/R0t,we transform Eq.(2)to a one-dimensional chain of modified discrete FHN equations:
Here we assume that the total charge on the membrane of the myelinated axon (i.e., the charge stored by the nonlinear membrane capacitor) is governed by the polynomial function[6]
wherec0is the constant value of the membrane capacitance,αis the capacitive feedback parameter having dimensions of inverse voltage squared.Physically,unis the membrane potential at thenth excitable membrane site known as Ranvier node whilevnis the corresponding recovery variable.Diffusive solitons as carriers of energy are well understood in conservative media, but the situation here is different because the medium is highly dissipative with the nonlinear ionic current insufficient to balance the energy outflow due to dissipation.This generates additional nonlinearity by spontaneously inducing the nerve membrane to manage the ionic charges with theCVcharacteristics given by Eq.(4).This measure is to maintain a stable diffusive solitary wave profile in the myelinated axon.[25-27]Note that forα=0,Eq.(3)is reduced to the wellknown FHN equations.[11,12]
The recovery variablevndepicts the slow dynamics because the parametersa,b,c ≪1.For Eq.(3a), the first term,un, accounts for the positive feedback, where depolarization enhances more depolarization through the voltage-gated sodium channel.The second term,-u3n/3, is a rapid negative feedback loop, corresponding to the auto-inactivation of the sodium channel.The third term,-vn,represents a recovery process, which may be physically responsible for regulating the outward potassium currents that oppose depolarization.The last term is the discrete diffusive term with coupling strengthK, which is proportional to the difference in internodal currents through a given Ranvier node.The first term in Eq.(3b), i.e.,a, mainly measures the potassium leakage current,whilebuncaptures the activation of the voltage-gated potassium channel by the membrane potentialun, which increases the magnitude ofvn.Lastly,-cvncontrols the pumping of potassium ions out of the neuron.
Differentiating Eq.(3a) with respect to time and substituting the value of ˙vnfrom Eq.(3b)yield

System (5) is therefore the Li´enard form of the modified discrete FHN model of a myelinated nerve fiber.ForD0=D1=0 andα=0, Eq.(5) becomes the cubic Li´enard equation with linear damping and it is sometimes regarded as a generalization of damped oscillations.Furthermore, within the linear regime and when the dissipation is neglected,we obtain the well-known linear harmonic oscillator that finds numerous applications in both classical and quantum physics.The Li´enard type of equations have been extensively investigated from both mathematical and physical perspectives,and their study remains an active field of research in mathematical physics.[4,5,28-30]
Since Eq.(5) is non-integrable, we proceed it by using a perturbation technique to find appropriate solutions.In this light, all the dissipative coefficientsγ2andD1are perturbed to the orderε2because of minimal damping,[5]whereε ≪1 is a slow variable parameter.Also, we greatly minimize the rate at which the leakage potassium ions are discharged from the neuron by perturbingγ0to orderε3.Consequently keeping just terms up to orderε2,Eq.(5)is rewritten as
Frequency limits exist within which normal propagation of nerve impulse signals is monitored across the modified discrete FHN axon.In order to identify the exact range of frequency, we introduce a perturbation method where a suitable solution of the modified FHN model containingεparameter is exploited.Upon substitution of this solution into the modified diffusive FHN model(6),the wave dispersion relation is obtained with terms at order ofε0eiθn.Hence,we consider a simplified solution of our modified discrete FHN model(6)of the form
withθn=qn-ωt,whereωis the angular frequency andqis the normal mode wave number.
In the perturbation approach known as the multiple scale expansion in the semidiscrete approximation,ψ(n,t) is supposedly independent of the “fast” variablestandn.Instead,it depend on the “slow” variables defined byXi=εixandTi=εit, fori ≥1.A continuum limit approximation is then employed with the wave amplitudes,while the discrete nature of the phase is maintained.Interested readers are advised to consult[5,31]for a comprehensive coverage of this perturbation technique.

Fig.2.Linear dispersion curve of the nerve impulse for D0=0.04 and Ω20 =0.032.There is a lower cutoff mode q=0 with frequency ω0 =Ω0 and upper cutoff mode q=π with frequency ωmax=(Ω20+4D0)1/2.
Collecting terms at orderε0eiθngives the dispersion relation
which is plotted in Fig.2.From Eq.(8), the linear spectrum has a gapωmin=Ω0and it is limited by the cut-off frequencyωmax=(Ω20+4D0)1/2due to discreteness.
Terms of orderε1eiθngives
wherevg=D0sinq/ωis the group velocity of the linear wave packets.
Finally,we collect terms proportional toε2eiθnto have
By considering the reference mobile frameξi=Xi-vgTiandτi=Tiwithvgbeing the group velocity of the wave,we obtain
wherePis the real dispersion coefficient,QrandQiare respectively the real and imaginary parts of the nonlinear coefficient,whileRis the dissipative coefficient.These parameters are given by
Equation (11) is the CGL equation, and broadly speaking, it represents one of the most-studied nonlinear equations in the scientific community today.This is because it gives a qualitative and quantitative description of a myriad of physical activities.[4,32,33]For example,in a diffusive Hindmarsh-Rose neural network,the propagation of modulated nerve impulses observed is also governed by the CGL equation,which clearly demonstrates how neurons participate in processing and sharing of information.[5]
The capacitive feedback parameter,α,clearly influences the nonlinearity of Eq.(11).ForQi>0 andQi<0, we are dealing with the nonlinear gain and nonlinear loss,and the dynamics of the system is still governed by the CGL equation.[4]WhenQivanishes,the value ofαis given by

Modulational instability is generally considered within our current context as a precursor for the generation of localized modulated nerve impulses in the myelinated axon.Consequently,we can now study perturbationsε(ξ1,τ2)≪1 of the continuous wave (cw) solution to the CGL Eq.(11) by using the ansatz[39,40]
We substitute this ansatz into the CGL Eq.(11), linearize inε, and look for solutions of the formε(ξ1,τ2) = [¯a(τ2)+i¯b(τ2)]cos(¯qξ1), which correspond to a weak perturbation of the intense cw background.Note that ¯qis the constant wavenumber of the perturbation and we obtain a linear system for ¯a(τ2)and ¯b(τ2)as


as depicted in Fig.3.The band of ¯qcrexponentially decreases with an increase in the dissipation of the medium,i.e.,the initial growth of modulational perturbations around the cw solution is being suppressed with increase in dissipation and time.

Fig.3.Variation of the critical wavenumber qcr with dissipation R,for P=Qr=1.00.


Fig.4.Product PQr of coefficients in Eq.(12)as functions of wave number q and capacitive feedback parameter α,for D0=D1=0.02,Ω20 =0.032,γ1=-0.15,γ2=0.001,c=1/3: (a)three-dimensional plot,(b)the corresponding contour plot,(c)α <αcr,(d)α =αcr,(e)α >αcr.
We have so far defined the behavior of the cw solutions whenη2(τ2)>0,η2(τ2)=0 andη2(τ2)<0.These processes capture and crystallize the stability dynamics of the system,which clearly tie in with the Benjamin-Feir criteria.The big problem now is to rigorously look for the most appropriate solution to Eq.(16)that matches exactly with all these predictions.In this light, we substitutex=exp[-Rτ2/2]and transform Eq.(16)into
whereσr=4Qr/R,σi=4Qi/Randθ2=P¯q2/4Qr.Equation(19)is the modified Bessel equation, and for the special case ofα=αcrwe haveσi=0,leading to solution[39]
whereIiσrθ2is the modified Bessel function withIiσrθ2'denoting differentiation with respect to the argument.Asτ2→∞,solution in Eq.(20) reduces to ¯a(τ2)≈cos(σrθ2Rτ2/2+φ),
whereφis the phase difference.Becauseσrθ2Rτ2/2 =P¯q2τ2/2,it shows that solution(20)is most appropriate.
Figures 4(a) and 4(b) depict the three-dimensional plot of productPQrand the corresponding contour plot, as functions of wave numberqand capacitive feedback parameterα.It clearly captures and crystallizes regions of nonlinear excitations in the myelinated axon.In Fig.4(c) it is shown that forα<αcr,an increase in the wave numberqmainly favors the stability of plane waves in the myelinated axon.Figure 4(d) equally shows the same trend as in Fig.4(c), withα=αcr=1.00.The influence ofαon the stability of plane waves is clearly visible in Fig.4(e), whereα>αcr.For the caseα=1.25, once the critical wave number is exceeded, it becomes impossible to identify localized modes.However,an increase inαwith the case ofα=2.00 favors the generation of more localized modes,even when the wave number exceeds its critical value.These observations are consistent with the predictions made by Benjamin and Feir in fluid dynamics.[38]
The stability analysis just performed confirms the presence of localized wave packets in the myelinated axon,which we now proceed to analytically obtain the appropriate solutions.As the capacitive feedback parameterαis varied, we will consider two distinct solutions to the amplitude equation of motion.
3.1.Modulated impulses for α/=αcr and wavenumber q>0
Forα/=αcrand wavenumberq>0, the form of the solution to Eq.(11) that depicts modulated nerve nerves in the myelinated axon is given by[5,34,41,42]

where
A detailed analysis of spatial evolution of the modulated nerve impulses fort=0,20.0,40.0 is highlighted in Fig.5.Generally the form of the modulated nerve impulse is modified as time increases, with variation inαhaving minimal effects on this form.However,the amplitude of the modulated nerve impulse is greatly altered with variation inα.This is glaring with the transition ofαfrom 0.99 to 1.50 in Fig.5.This result suggests that the capacitive feed back parameterαis inextricably linked to the amplitude of the modulated nerve impulses in the myelinated axon,and ties in with the result obtained by Dikand´eet al.[6]
It should be noted that the spatial profile of the modulated nerve impulse solution (22) cannot be traced at lower cut off modeq=0.Furthermore,solution(22)remains undefined forα=αcr.Consequently we now seek for a specific solution in which these parameter values are valid, in order to fully account for the evolution of the modulated nerve impulses in the myelinated axon.

Fig.5.Evolution of modulated impulses according to Eq.(22)with ψ0=1.00,D0=D1=0.02,Ω20 =0.032,γ1=-0.15,γ2=0.001,c=1/3,q=ε =1.00,for various α.
3.2.Modulated impulses for α =αcr and at lower cut off mode(q=0)
Forα=αcrand at lower cut off mode(q=0),the form of the envelope soliton solution of Eq.(11), according to Ref.[37],is given by
whereX=X(ξ1,τ2)=ς0(τ2)ξ1+β0(τ2),withKbeing a real constant,χ(τ2),ς0(τ2),β0(τ2),andρ(τ2)are real functions of variableτ2to be defined anduis a real function of variableX.Inserting the ansatz(23)into Eq.(11)and separating the real and imaginary parts give


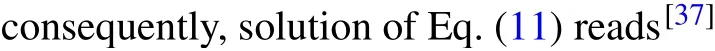
It is important to note thatρ0is an arbitrary real constant and we have setς00=1.00 without loss of generality.
We now show how the modulated nerve impulses propagate in the myelinated axon by substituting Eq.(33a) into solution(7)of the nerve impulse.We fix the valuesR=2.00,ψ0=1.00,K=20.0,K0=ρ0=0.00,Qr=2.00 andP=1.00 without loss of generality,but just to simplify the mathematical computations.This leads to
Recall thatξ1=ε(x-vgt),τ2=ε2t,x=nand at lower cutoff modeq=0,ω=Ω0,vg=0.This reduces solution(34)to the form
A plot of the spatial profile of the nerve impulses in the myelinated axon with the variation of the perturbation parameterεis given in Fig.6 at different time frames.It is also observed that the modulated impulses become more localized as the perturbationεincreases.For example, forε=0.100 as in the first diagram of Fig.6,the modulated impulses overlap,indicating that neuronal information is slowly transmitted in the myelinated axon.However, as the perturbation gradually increases toε=1.000 as in the last diagram of Fig.6,the neural signals are transmitted with high efficiency.This observation suggests that the flow of ionic charges within the neural membrane is greatly influenced by the variation of the perturbation parameterε.
Analysis of the time series dynamics of the nerve impulse fixed at the origin generates the curves in Fig.7.Also,as the perturbation increases, the nerve impulses gradually become more localized,suggesting that these perturbations physiologically affect the opening and closing of voltage-gated ion channels in the nerve membrane.In particular, forε=1.000, we observe a single spike which is qualitatively similar to those recorded in squid axons.[40]

Fig.6.Evolution of modulated impulses at lower cutoff mode(q=0),according to Eq.(35)with Ω0=0.18.The perturbation ε is varied.

Fig.7.Time series dynamics of the nerve impulse according to Eq.(35),when the perturbation parameter ε is varied.This is at lower cutoff mode q=0 and fixed at the origin(n=0)for Ω0=0.18.
The possibility of different outcomes with collision of nerve impulses in neural networks has greatly motivated the investigation of this phenomenon in this section.Action potentials are considered to be an electrical phenomenon,which is governed by the theoretical equations initially proposed by Hodgkin and Huxley.[10]Despite the numerous successes recorded by the HH model, it has been recently subjected to intense criticism.For example, it has been suggested that action potentials are a class of nonlinear waves called solitons,[43]which upon head-on collision, two solitons penetrate and continue to move with undistorted profile.Furthermore, penetration of two solitons propagating in a lipid bilayer membrane was also predicted in Ref.[43].However, a plethora of results[44-46]clearly indicates that pulse penetration is not observed in excitable membranes.Considering this evidence, it is unlike that action potentials are correctly described as solitons.It must be underscored that this does not cast doubt on the perception that an action potential is a nonlinear acoustic phenomenon.Other solitary waves like in Bose-Einstein condensates[47]and catalytic surface reactions,[48]annihilate upon head-on collision.Interestingly, annihilation however seems to be a comparatively rare scenario, suggesting that this phenomenon may require special type of nonlinearity in the medium of interaction.Furthermore, there are strong indications that excitable cells reside close to a phase transition.[49,50]If the latter incorporates the nonlinearity that allows for the generation of an action potential,the pulse could entail a phase change of the excitable medium.This transient realization of a different material phase may lead to pulse annihilation when two action potentials meet.In order to be able to penetrate, the pulses would have to propagate through this new phase.
4.1.Localized standing waves
We now numerically integrate Eq.(6), which is the Li´enard form of the modified discrete FHN equation.This is achieved by using the fifth-order Runge-Kutta scheme with a time step of 0.01, on a spatial domain supportingN=101 dynamical units,under periodic boundary conditions.To solve this non-integrable discrete nonlinear equation,we take,as initial condition, the analytical solution given by the modulated nerve impulse solution(22),which att=0.00 reads
On the upper part of Fig.8 for the given set of the system parameter values, it is observed that after sending the pulse ansatz in the system, the structure preserves this excitation state permanently with no event of propagation.This suggests that stationary localized pulses are accessible for our biological system.This solution is indeed an asymmetric localized solution which intimates the existence of a stable stationary bright envelope soliton solution for our modified discrete FHN model equation (6).Figures 8(a), 8(b), and 8(c)show the three-dimensional spatiotemporal display,the upper view, and the profile of this static pulse solution at all times,respectively.
Under other parameter conditions, this pulse solution turns into a localized standing wave.These are highlighted in Figs.8(d)-8(f).Figure 8(f) shows the standing wave profiles at different times.Further variations in the value of nonlinear coefficientγ1lead to different profiles of localized standing waves as reflected in Fig.9.

Fig.8.Membrane potential solutions of the modified discrete FHN Eq.(6) under two different parameters conditions.The upper figures depict a stationary localized pulse:(a)three-dimensional view,(b)upper view,(c)profile at all times.The parameters are taken as c=q=ε=1.00,α=0.0001,ψ0 =1.00, γ1 =0.05, γ2 =721.0, Ω20 =0.320, D0 =0.002, D1 =0.002.The lower figures depict a spatially localized standing wave: (d) threedimensional view, (e) upper view, (f) profiles of the wave at different times.The parameters values are the same as those in the upper figures, but γ2=0.00 and Ω20 =1.49.

Fig.9.Effects of variation in γ1 on the localized waves of the modified discrete FHN Eq.(6).The parameters are same as those in Fig.8 but for(a)γ1=0.001,(b)γ1=0.003,(c)γ1=0.045,and(d)γ1=0.050.
4.2.Propagation and collision
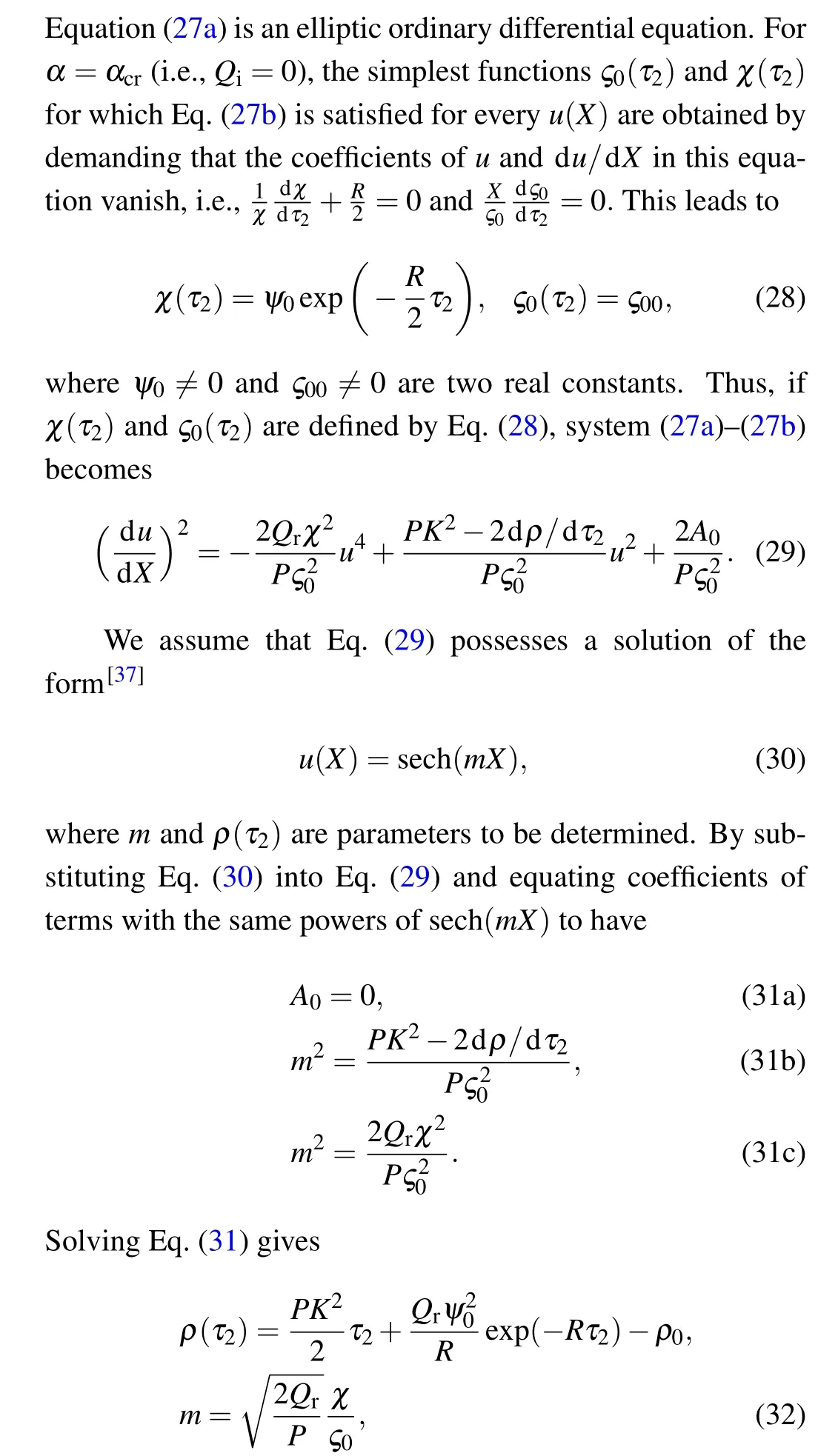
By varying the wave dispersion coefficientD0in the modified FHN neural network,we observe the profile of the waves as shown in Fig.10.
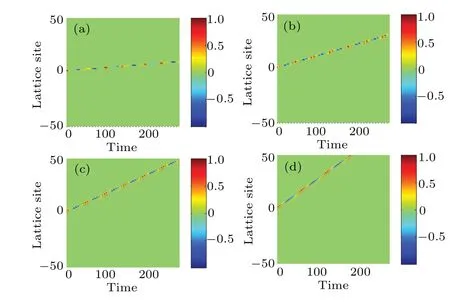
Fig.10.Effect of dispersion coefficient D0, on the propagation of action potential in the modified FHN model.The parameters are same as those in Fig.8 but for(a)D0=0.02,(b)D0=0.04,(c)D0=0.06,and(d)D0=0.08.
It is generally found that an increase inD0leads to a corresponding increase in the speed of propagation of nerve impulses.For the particular case in Fig.10(d),the nerve impulse takes just about 165 to move across the network.Based on these observations, we now plot the time of propagationτpand the propagation speedvp,as a function ofD0,as shown in Fig.11.
Figure 11(a)clearly shows that the time of propagationτpis inversely proportional to the dispersion coefficientD0,while Fig.11(b)depicts that the propagation speedvpincreases exponentially withD0.We further plot the spatial profiles of the nerve impulses at a particular value of dispersion coefficientD0,as shown in Fig.12.The spatial profiles of the nerve impulses in Fig.12(c)give the evolution from a pulse impulse att=0.00 to an asymmetric impulse att=206.4.

Fig.11.(a)Behavior of τp that is inversely proportional to D0,(b)behavior of vp that increases exponentially with D0.
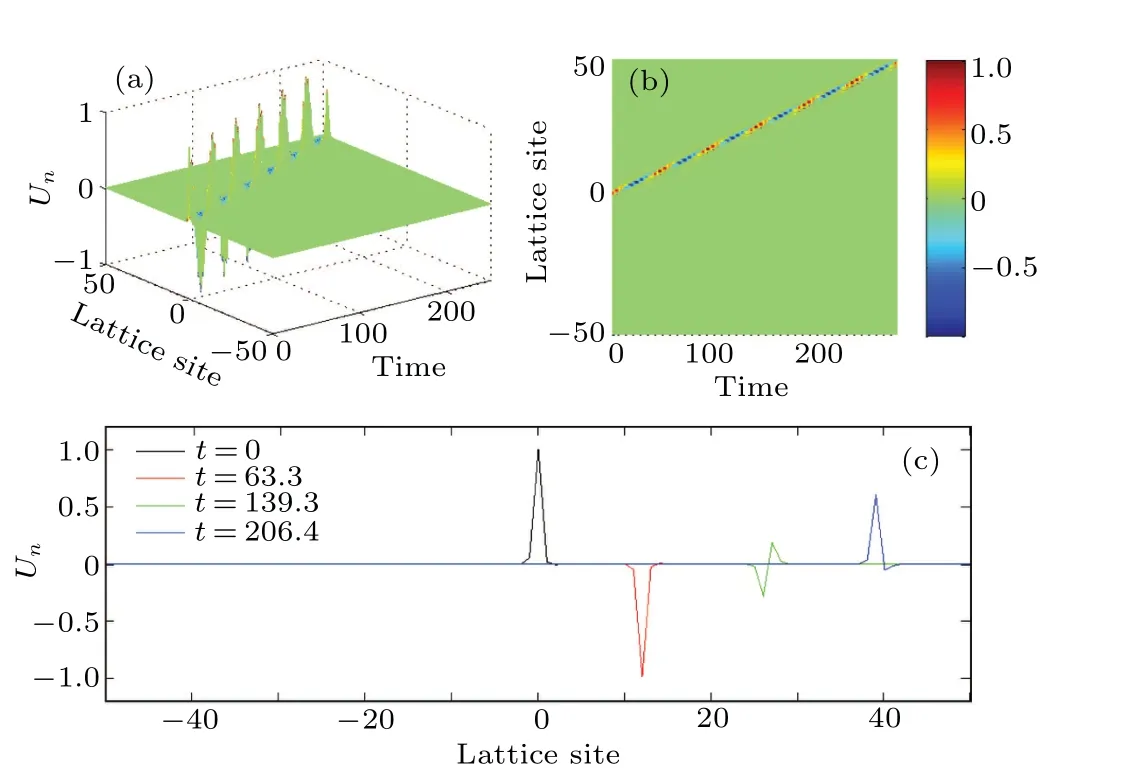
Fig.12.Propagation of the nerve impulses for dispersion coefficient D0 =0.06, as in Fig.10(c): (a) spatiotemporal evolution of nerve impulse,(b)the corresponding contour plot of the nerve impulse,(c)spatial profiles of the nerve impulse at different times.
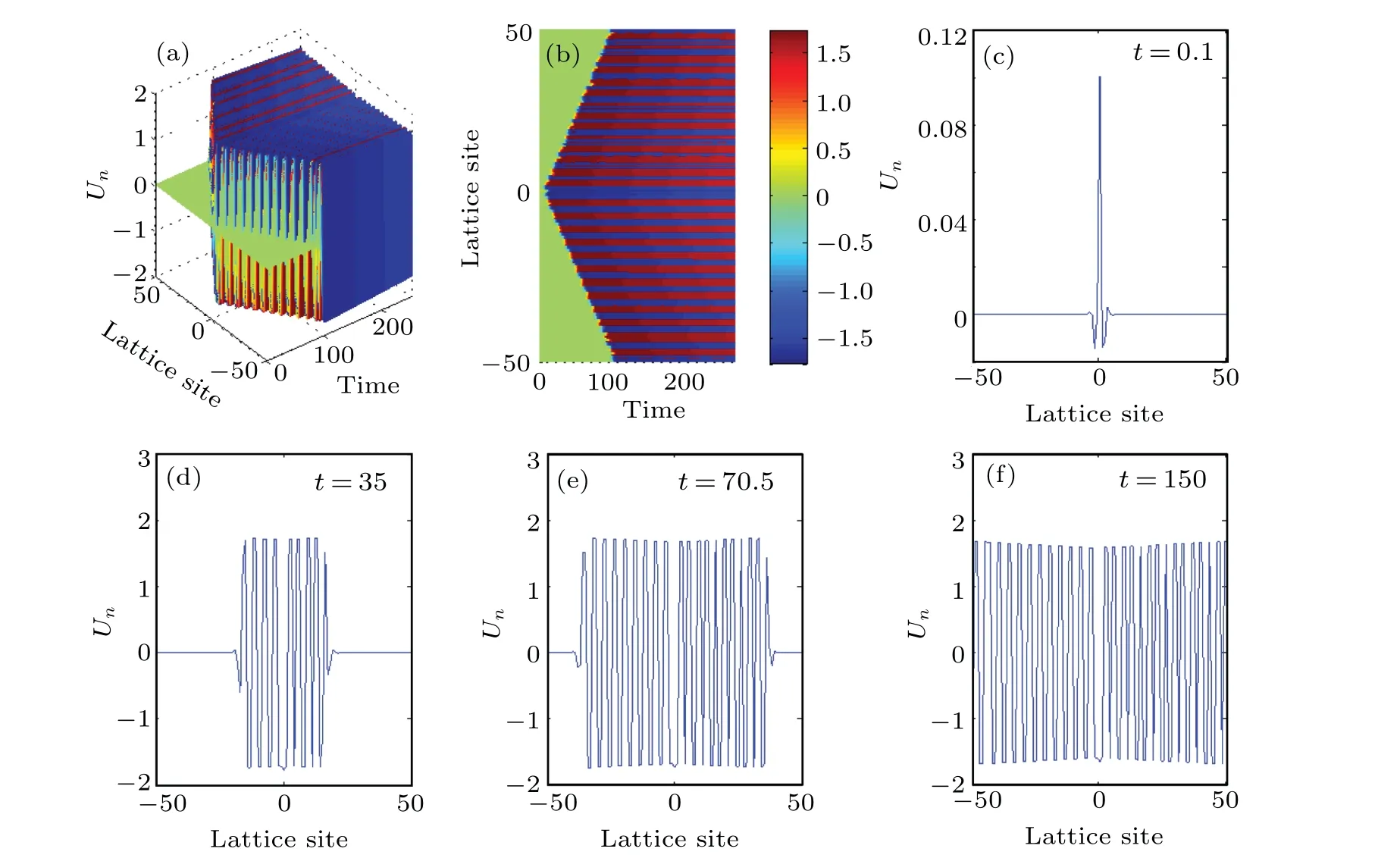
Fig.13.Collision of bi-pulse solution of the modified FHN Eq.(6):(a)three-dimensional view,(b)upper view,(c)profile at t=0.1,(d)profile at t=35,(e)profile at t=70.5,(f)profile at t=150.
By further choosing suitable parameters in the numerically integration of the modified discrete FHN Eq.(6), interesting behavior of the nerve impulse signal is revealed as in shown Fig.13.The pulse degenerates into a bi-soliton which propagates in opposite directions within the lattice of the myelinated axon.As a result of the imposed periodic boundary conditions, these two pulses collide at the boundaries of the neuronal lattice and annihilate each other.[1]Immediately after the annihilation,a dynamic structure known as multipulse crystal emerges in the myelinated axon.This multipulse solution is indeed a quadripulse,which can be viewed as being indicative of a localized firing state,with the occurrence of spatially localized static spiking structures in the neuronal network as in Fig.13(d) whent=35.These nerve impulse structures eventually evolve to occupy the entire lattice site as depicted in Fig.13(f)whent=150.
Soliton-like modes have already been identified in neural networks.For example, Moukamet al.reported on localized nonlinear excitations in diffusive Hindmarsh-Rose(HR) neural networks with nearest-neighbor linear electrical couplings.[5]On the other hand, Mvogoet al.considered the same HR network but with long-range diffusive coupling.[51]In both analyses, the neural membrane capacitance was assumed to be constant.However Dikand´eet al.considered a nonlinear membrane capacitance for both myelinated and unmyelinated nerve fibers, and showed that nerve impulses are well-localized in neural networks.[6]Our current investigation for single mode oscillations mainly deals with the modified FHN model.In fact we have considered an electrical lattice in the form of a nonlinear transmission line that models the modified discrete FHN system, in which the charge stored in the membrane,Q, is a nonlinear function of the trans-membrane voltage, i.e.,Q(un)=c0(1-αu2n)un.This nonlinear membrane capacitance is spontaneously induced during the generation and transmission of nerve impulse in the highly dissipative myelinated axon.The capacitive nonlinearity coupled with that of the ionic current dynamically competes with the highly dissipative effects experienced in the myelinated axon.By using the multiple scale expansion in the semi-discrete approximation,we have shown that the system of equation governing the propagation of modulated nerve impulses in the dissipative myelinated axon is the CGL amplitude equation.This equation is reduced to the DNLS equation at the critical value of the capacitive feedback parameter (i.e., atα=αcr).We equally investigate the modulational instability of the propagating nerve impulses, and confirm the predictions made by Benjamin and Feir in fluid dynamics.[38]The capacitive feedback parameterαplays a crucial role in plane wave stability.In fact, we find that the dissipation generally suppresses the modulational instability by switching the growth of the perturbations into an oscillatory behavior.This is graphically demonstrated in Fig.3,where the critical wavenumber ¯qcrdecreases with an increase in the dissipationR.The amplitude of the propagating modulated nerve impulses is generally altered with variation inα,suggesting that this parameter has a physiological effect on the ion channels in the membrane.Forα=αcr, the modulated nerve impulses are also identified in the myelinated axon(in both the spatial and time domains)at lower cutoff mode, which become more localized as the perturbation parameterεis increased.The results of numerical simulations of the modified discrete Fitzhugh-Nagumo equation show the propagation, collision, and annihilation of impulses in the neural network.
The ideal form of theC-Vcharacteristic is actually dictated by reliable experimental data on nerve impulse transmission under appropriate physiological conditions.However,an in-depth analysis involving available experimental data on the response of the nerve membrane capacitance to the transmembrane excitations is vital to gain complete qualitative as well as quantitative understandings of the nerve impulse generation and transmission in a myelinated axon with self excitable membrane.In this light, we believe that under appropriate physiological conditions, experimental investigations will shed more light on our current theoretical study carried out on a myelinated axon with self-excitable membrane.
Appendix A
Acknowledgements
The research activity carried out on neural dynamics with nonlinear membrane capacitance by Professor A.M.Dikand´e,greatly motivated N.Oma Nfor to initiate this project.
相关热词搜索:waves myelinated Localized
- 范文大全
- 说说大全
- 学习资料
- 语录
- 生肖
- 解梦
- 十二星座
-
主题党日活动交流发言8篇
主题党日活动交流发言8篇主题党日活动交流发言篇13月13日,东城区党史学习教育动员大会召开。市委
【活动总结】 日期:2022-12-23
-
2022年4月主题党日活动记录范文15篇
2022年4月主题党日活动记录范文15篇2022年4月主题党日活动记录范文篇1一个崇尚阅读的民族,必然精神饱满、意气风发、活力四射。习近平总书记强调:“学习
【活动总结】 日期:2022-08-01
-
家乡赋|最美的家乡赋
家乡赋 孙传志 今安康市,白河双丰镇,吾之家乡也。三环沃土,山水环抱。其北依山,山系五岭,山
【调研报告】 日期:2020-04-01
-
少先队的光荣历史故事 队前教育-光辉历程
2017-2018学年队前教育1光辉历程一、劳动童子团1924——1927二、三十年代年的中国是一个
【法律文书】 日期:2020-06-23
-
党支部1-12月全年主题党日活动计划表
2022年党支部主题党日活动计划表序号活动时间活动方式活动内容12022年1月专题学习研讨集中观看2022年新年贺词,积极开展学习研讨交流。组织生活会组织党员认真对照党章...
【活动总结】 日期:2022-10-14
-
【人教版1-6年级数学上册知识点精编】1-6年级数学人教版教材
人教版二年级数学上册知识点汇总第一单元长度单位一、米和厘米1、测量物体的长度时,要用统一的标准去测量
【调研报告】 日期:2020-11-08
-
2022年2月份主题党日活动记录5篇
2022年2月份主题党日活动记录5篇2022年2月份主题党日活动记录篇1尊敬的党组织:在今年的开学初,本人积极参加教研室组织的教研活动,在学校教研员的指
【活动总结】 日期:2022-08-12
-
医院最佳主题党日活动11篇
医院最佳主题党日活动11篇医院最佳主题党日活动篇1 医院最佳主题党日活动篇2为隆重纪念中国共产党成立100周年,进一步巩固党的群众路线教育实践活动成果,切实
【活动总结】 日期:2022-10-29
-
2023年平安校园建设方案13篇
平安校园建设方案“平安校园”创建工作,我们幼儿园全体教职员工一直把它当作头等大事来抓。领导高度重视,以“平安校园”创建活动为抓手,建立和规范校园安全工作机制
【规章制度】 日期:2023-11-02
-
主题党日活动记录202210篇
主题党日活动记录202210篇主题党日活动记录2022篇12021年是中国共产党成立100周年,为广泛开展爱国主义宣传教育,铭记党的历史,讴歌党的光辉历程,
【活动总结】 日期:2022-08-02
-
正式的晚宴邀请函 公司晚宴邀请函
尊敬的先生 女士: 我公司谨定于xxxx年xx月xx日xx:xx在xxxx店隆重举行xx市xx届xxxx晚宴(宴会地址:xx区xx路xxxx) 敬请届时光临!xxxxxx集团股份有限公司xxxx有限公司敬邀xxxx年xx月xx日
【简历资料】 日期:2019-08-03
-
一年级新学期目标简短_一年级学生新学期打算
新学期到了,我是一年级下册的小学生了。 上课的时候,我要认真学习,不做小动作,认真听讲。我要认真学习,天天向上,努力学习,耳朵要听老师讲课,眼睛要瞪得大大的看老...
【简历资料】 日期:2019-10-26
-
《国行公祭,为佑世界和平》课文原文阅读_国行公祭为佑世界和平每段段意
国行公祭,为佑世界和平钟声“国行公祭,法立典章。铸兹宝鼎,祀我国殇。”侵华日军南京大屠杀遇难同胞纪念
【简历资料】 日期:2020-11-28
-
[信访复查复核制度作用探讨]信访复查复核有用吗
作为我国特有的一项制度,信访制度的出现并长期存在不是偶然的,虽然一些法学专家认为信访制度具有“人治”
【职场指南】 日期:2020-02-16
-
[党员干部2019年主题教育个人问题检视清单及整改措施2篇] 党员干部
2019年主题教育问题检视清单及整改措施根据主题教育领导小组办公室《关于认真做好主题教育检视问题整改
【求职简历】 日期:2019-11-08
-
网络维护工作内容_(精华)国家开放大学电大专科《网络系统管理与维护》形考任务1答案
国家开放大学电大专科《网络系统管理与维护》形考任务1答案形考任务1理解上网行为管理软件的功能【实训目
【职场指南】 日期:2020-07-17
-
红旗颂朗诵稿原文【《红旗颂》朗诵词】
《红旗颂》朗诵词 女:晴空万里,红旗飘扬, 六十载风云,我们昂首阔步。 男:六十个春秋,
【职场指南】 日期:2020-02-16
-
党委会与局长办公会的区别_局长办公会制度
为进一步加强xxx局工作的规范化、制度化建设,提高行政效能,规范议事程序,特制定本制度。一、会议形式1、局长办公会议由局长、副局长参加。由局长召集和主持。根据工作需要...
【求职简历】 日期:2019-07-30
-
《铁拳砸碎“黑警伞”》警示教育片观后感
影片深刻剖析了广西北海市公安局海西派出所原所长张枭杰蜕变堕落的轨迹。观看警示教育片后,做为一名党员教
【简历资料】 日期:2020-08-17
-
如何凝心聚力谋发展【坚定信心谋发展凝心聚力促跨越】
当前,清河正处于在苏北实现赶超跨越基础上全面腾飞的战略机遇期,处于在全市率先实现全面小康基础上率先实
【简历资料】 日期:2020-03-17
-
课题研究报告3篇
课题研究报告3篇课题研究报告篇1十九大以来,组织部门主动适应全面从严治党新形势新要求,在加强自身政治文化建设方面做了大量工作,取得显著成效。下面是星星阅读网
【其他范文】 日期:2022-08-19
-
[婚庆主持串词]最新结婚主持词
导读:婚丧嫁娶范文,婚庆主持串词在线阅读,教你怎么写,格式什么样,科教范文网提供各种参考范例: 炮竹声声贺新婚,欢声笑语迎嘉宾 尊敬各位来宾,各位领导,各位 It...
【礼仪】 日期:2019-10-04
-
2022年在全市“转作风优环境”活动年动员大会上的讲话
下面是小编为大家整理的2022年在全市“转作风优环境”活动年动员大会上的讲话
【其他范文】 日期:2022-08-17
-
竞聘答辩需要注意的事项 竞聘答辩
Vdt287{display:none;}1、充分准备是必不可少的。把你自己的所学、所做,全部展示出来,才能获取更多的分值。如果你有打印的文稿,一定要准备的数量尽量多一点。(呵呵,这...
【其他范文】 日期:2019-09-14
-
_《登顶之舞》学习心得
导读:讲话稿范文,《登顶之舞》学习心得在线阅读,教你怎么写,格式什么样,科教范文网提供各种参考范例:《登顶之舞》学习心得《登顶之舞》是用中国人的哲学来写,用中国人...
【口号大全】 日期:2019-10-05
-
[在第一届纪律检查委员会第二次全体会议上的工作报告]中央纪律检查委员会地址
同志们:我代表中共西安市鄠邑区第一届纪律检查委员会常务委员会向第二次全体会议作工作报告,请予审议。这
【其他范文】 日期:2020-01-13
-
贯彻执行中央八项规定精神和省州县相关规定情况报告
贯彻,汉语词语,拼音是guànchè,意思是彻底实现或体现,以下是为大家整理的关于贯彻执行中央八项规定精神和省州县相关规定情况的报告4篇,供大家参考选择。贯彻执行中央八项...
【其他范文】 日期:2023-01-04
-
2022在县政协资政会上讲话【完整版】
各位常委、同志们:很高兴来参加政协资政会。今天的资政会以打造智造强县,推动高质量发展为主题,选题精准、重点突出、安排及时,有力呼应了县委、县政府的重点工作部署,充...
【其他范文】 日期:2022-09-16
-
2013-2014学年上学期大学辅导员工作总结:辅导员个人工作总结
北区熟悉的环境,学生们清晰的面庞,同事们坦诚的相待,领导们真诚的关怀,一切都是那么相似,那么明晰,但我们将要面对的却又如此的不同,时间在改变,学生在成长,我和可爱...
【导游词】 日期:2019-08-01
-
秋季运动会开幕词范文精选五篇
【学习啦--开闭幕词】 运动会指体育运动的竞赛会,有奥运会等大型运动会,只是范围不同。最早的运动会
【汇报体会】 日期:2020-01-13
-
理论中心组学习总体国家安全观发言材料9篇
理论中心组学习总体国家安全观发言材料9篇理论中心组学习总体国家安全观发言材料篇1(八)深入学习贯彻中央以及省的重要会议和文件精神深入学习贯彻年度内中央以
【发言稿】 日期:2022-08-04
-
军转座谈会交流发言4篇
军转座谈会交流发言4篇军转座谈会交流发言篇1大家好,我叫贺丽,2015届选调生,来自康定市委组织部,现在省委编办跟班学习。今天,非常荣幸向大家汇报我的学习收
【发言稿】 日期:2022-10-27
-
12岁生日小寿星发言4篇
12岁生日小寿星发言4篇12岁生日小寿星发言篇1各位来宾、各位朋友:大家好!今天,我们欢聚在这里,共同庆祝**十二周岁生日。首先,我代表**的父母以
【发言稿】 日期:2022-07-31
-
党内警告处分表态发言14篇
党内警告处分表态发言14篇党内警告处分表态发言篇1尊敬的各位领导、同事们:大家上午好!刚才会上宣布了党委关于我任职的决定,我首先衷心感谢党委的信任和
【发言稿】 日期:2022-09-13
-
【企业疫情风险控制方案】 2020企业复工疫情方案
企业疫情风险控制方案2020新冠病毒肺炎疫情防控工作总结汇报3篇 关于新型冠状病毒感染的肺炎疫
【演讲稿】 日期:2020-02-27
-
党内警告处分党员讨论发言3篇
党内警告处分党员讨论发言3篇党内警告处分党员讨论发言篇1大家好!作为新时期的一名大学生,认真学习、深刻领会、全面贯彻省党代会精神,是当前和今后一个时期重
【发言稿】 日期:2022-08-07
-
廉政大会总结发言稿7篇
廉政大会总结发言稿7篇廉政大会总结发言稿篇1各位领导,同志们:根据会议安排,我就党风廉政建设工作做表态发言,不妥之处,请批评指正。一、提高认识,切实
【发言稿】 日期:2022-10-30
-
被约谈的表态发言8篇
被约谈的表态发言8篇被约谈的表态发言篇1各位领导、各位党员大家好:这天我能站在鲜红的党旗下,
【发言稿】 日期:2022-12-24
-
破冰提能大讨论个人发言4篇
破冰提能大讨论个人发言4篇破冰提能大讨论个人发言篇1党史学习教育开展以来,我坚持读原著、学原文、悟原理。今天,根据会议安排,现在我就“学史明理”主题谈几点个
【发言稿】 日期:2022-10-09
-
巡察整改专题民主生活会总结发言8篇
巡察整改专题民主生活会总结发言8篇巡察整改专题民主生活会总结发言篇1按照区委统一部署和纪监委、巡察办关于召开党史学习教育专题组织生活会的工作安排,近期我紧贴
【发言稿】 日期:2022-10-12
-
2023年中国行政区划调整方案(设想优秀3篇
中国行政区划调整方案(设想优秀民政部第二次行政区划研讨会会议内容一、缩省的意义与原则1.意义1)利于减少中间层次中国行政区划层级之多为世界之最,既使管理成本
【周公解梦】 日期:2024-02-20
-
学习周永开先进事迹心得体会3篇
学习周永开先进事迹心得体会【一】通过学习周永开老先生先进事迹后,结合自己工作思考,感慨万千。同样作为
【格言】 日期:2021-04-10
-
2023年和儿媳妇在一起幸福的句子3篇
和儿媳妇在一起幸福的句子1、假如人生不曾相遇,我还是那个我,偶尔做做梦,然后,开始日复一日的奔波,淹没在这喧嚣的城市里。我不会了解,这个世界还有这样的一个你
【格言】 日期:2023-11-10
-
最满意的三项工作200字【最新党办公务员副主任提拔考察个人三年思想工作总结报告】
党办公务员个人三年工作总结近三年来,本人在组织、领导的关心指导和同事们的团结协作下,尽快完成主角的转
【格言】 日期:2021-02-26
-
XX老干局推进党建与业务深度融合发展工作情况调研报告:党建调研报告
XX老干局推进党建与业务深度融合 发展工作情况的调研报告 党建工作与业务工作融合发展始终是一个充满生
【成语大全】 日期:2020-08-28
-
中国共产党第三代中央领导集体的卓越贡献
中国共产党第三代中央领导集体的卓越贡献 --------------继往开来铸就辉煌 【摘要】改
【成语大全】 日期:2020-03-20
-
信息技术2.0能力点 [全国中小学教师信息技术应用能力提升工程试题题库及参考答案「精编」]
全国中小学教师信息技术应用能力提升工程试题题库及答案(复习资料)一、判断题题库(A为正确,B为错误)
【格言】 日期:2020-11-17
-
党建工作运行机制内容有哪些_构建基层党建工作运行机制探讨
党的基层组织是党在社会基层组织中的战斗堡垒,是党的全部工作和战斗力的基础。加强和改进县级以下各类党的
【经典阅读】 日期:2020-01-22
-
电大现代教育原理_最新国家开放大学电大《现代教育原理》形考任务2试题及答案
最新国家开放大学电大《现代教育原理》形考任务2试题及答案形考任务二一、多项选择题(共17道试题,共3
【成语大全】 日期:2020-07-20
-
集合推理_七,推理与集合
七推理与集合1 期中考试数学成绩出来了,三个好朋友分别考了88分,92分,95分。他们分别考了多少分
【名人名言】 日期:2020-12-18
-
关于三农工作重要论述心得体会3篇
关于三农工作重要论述心得体会3篇关于三农工作重要论述心得体会篇1习近平总书记指出:“建设现代化国家离不开农业农村现代化,要继续巩固脱贫攻坚成果,扎实推进乡村
【学习心得体会】 日期:2022-10-29
-
【福生庄隧道坍塌处理方案】 福生庄隧道在哪里
(呼和浩特铁路局大包电气化改造工程指挥部,内蒙古呼和浩特010050)摘要:文章介绍了福生庄隧道
【学习心得体会】 日期:2020-03-05
-
五个一百工程阅读心得体会13篇
五个一百工程阅读心得体会13篇五个一百工程阅读心得体会篇1凡益之道,与时偕行。在全国网络安全和信
【学习心得体会】 日期:2022-12-07
-
城管系统警示教育心得体会9篇
城管系统警示教育心得体会9篇城管系统警示教育心得体会篇1各党支部要召开多种形式的庆七一座谈会,组织广大党员进行座谈,回顾党的光辉历程,畅谈党的丰功伟绩,
【学习心得体会】 日期:2022-10-09
-
发展对象培训主要内容10篇
发展对象培训主要内容10篇发展对象培训主要内容篇1怀着无比激动的心情,我有幸参加了__新区区委党校20__年第四期(区级机关)党员发展对象培训班。这次的学习
【培训心得体会】 日期:2022-09-24
-
扶眉战役纪念馆心得体会11篇
扶眉战役纪念馆心得体会11篇扶眉战役纪念馆心得体会篇1有那么一段历史,低诉着血和泪的故事,慢慢地,随岁月老去;有那么一群人,放弃了闲逸的人生,辗转奔波中
【学习心得体会】 日期:2022-08-03
-
凝聚三种力量发展全过程人民民主心得体会12篇
凝聚三种力量发展全过程人民民主心得体会12篇凝聚三种力量发展全过程人民民主心得体会篇1新民主主义革命是指在帝国主义和无产阶级革命时代,殖民地半殖民地国家中的
【学习心得体会】 日期:2022-08-31
-
2022年全国检察长会议心得7篇
2022年全国检察长会议心得7篇2022年全国检察长会议心得篇1眼睛是心灵上的窗户,我们通过眼睛才能看到世间万物,才能看到眼前这美好的一切。拥有一双明亮的眼
【学习心得体会】 日期:2022-10-31
-
全面从严治党的心得体会800字7篇
全面从严治党的心得体会800字7篇全面从严治党的心得体会800字篇1中国特色社会主义是我们党领导
【学习心得体会】 日期:2022-12-14
-
教师全国两会精神学习专题研讨交流材料6篇
教师全国两会精神学习专题研讨交流材料6篇教师全国两会精神学习专题研讨交流材料篇1通过对两会精神深入系统的学习,作为新一代的青年人,更应该严格要求自己,贯彻落
【教师心得体会】 日期:2022-08-11
-
 2024年主题教育民主生活会批评与自我批评意见(38条)(范文推荐)
2024年主题教育民主生活会批评与自我批评意见(38条)(范文推荐)
2023年主题教育民主生活会六个方面个人检视、相互批评意见:1 理论学习系统性不强。学习习近平新时代中国特色社会主义思想不深不透,泛泛而学的时候多,深学细照的时候少,特...
【邓小平理论】 日期:2024-03-19
-
 2024年交流发言:强化思想理论武装,增强奋进力量(完整)
2024年交流发言:强化思想理论武装,增强奋进力量(完整)
习近平总书记指出:“一个民族要走在时代前列,就一刻不能没有理论思维,一刻不能没有思想指引。”党的十八大以来,伴随着新时代中国特色社会主义思想在实践中形成发展的历程...
【三个代表】 日期:2024-03-19
-
 2024年度镇年度县乡人大代表述职评议活动总结
2024年度镇年度县乡人大代表述职评议活动总结
xx镇20xx年县乡人大代表述职评议活动总结为响应县级人大常委会关于开展县乡两级人大代表述职评议活动,进一步激发代表履职活力,加强代表与人民群众的联系,提高依法履职水平...
【马克思主义】 日期:2024-03-19
-
 “千万工程”经验学习体会(研讨材料)
“千万工程”经验学习体会(研讨材料)
“千万工程”是总书记在浙江工作时亲自谋划、亲自部署、亲自推动的一项重大决策,也是习近平新时代中国特色社会主义思想在之江大地的生动实践。20年来,“千万工程”先后经历...
【三个代表】 日期:2024-03-19
-
 2024年在市政协机关工作总结会议上讲话
2024年在市政协机关工作总结会议上讲话
同志们:刚才,XX同志对市政协机关20XX年工作进行了很好的总结,很精炼,很到位,可以感受到去年机关工作确实可圈可点。XX同志宣读了表彰决定,机关优秀人员代表、先进集体代...
【邓小平理论】 日期:2024-03-18
-
 在全区防汛防涝动员暨河长制工作推进会上讲话提纲【完整版】
在全区防汛防涝动员暨河长制工作推进会上讲话提纲【完整版】
区长,各位领导,同志们:汛期已经来临,我区城区防涝工作面临强大考验,形势不容乐观。年初,区城区防涝排渍指挥部已经召开专题调度会,修订完善应急预案,建立网格化管理机...
【马克思主义】 日期:2024-03-18
-
 2024年镇作风整治工作实施方案(完整文档)
2024年镇作风整治工作实施方案(完整文档)
XX镇作风整治工作实施方案为深入贯彻落实党的二十大精神及省市区委深化作风建设的最新要求,突出重点推进干部效能提升,坚持不懈推动作风整治工作纵深发展,根据《关于印发《2...
【毛泽东思想】 日期:2024-03-18
-
 2024市优化法治化营商环境规范涉企行政执法实施方案【优秀范文】
2024市优化法治化营商环境规范涉企行政执法实施方案【优秀范文】
xx市优化法治化营商环境规范涉企行政执法实施方案为持续优化法治化营商环境,激发市场主体活力和社会创造力,规范行政执法行为,创新行政执法方式,提升行政执法质效,着力解...
【毛泽东思想】 日期:2024-03-18
-
 2024年度关于开展新一轮思想状况摸底排查工作通知(完整)
2024年度关于开展新一轮思想状况摸底排查工作通知(完整)
关于开展新一轮思想状况摸底排查工作的通知为深入贯彻落实关于各地开展干部职工思想状况大摸底大排查情况上的批示要求和改革教育第二次调度会议精神,有针对性做好队伍教育管...
【三个代表】 日期:2024-03-18
-
 2024年公路养护中心主任典型事迹材料(完整文档)
2024年公路养护中心主任典型事迹材料(完整文档)
“中心的工作就是心中的事业”——公路养护中心主任典型事迹材料**,男,1976年6月出生,1993年参加工作,2000年4月调入**区交通运输局工作,大学本科学历,中共党员,现任**...
【马克思主义】 日期:2024-03-17
